张纯喜研究员
中科院化学所光化学实验室
Tel: 010-82617053
E-mail: chunxizhang@iccas.ac.cn

个人简历:
1994年毕业于哈尔滨工程大学化学工程专业;1997年在中科院福建物构所获硕士学位,硕士期间从师于刘秋田研究员进行固氮酶化学模拟研究;2000年在中科院植物所获博士学位,博士期间从师于匡廷云院士进行光合作用研究;2000~2004年先后在瑞典Lund大学Prof. Stenbjorn Styring小组和法国CEA Saclay原子能研究中心Prof. A. William Rutherford小组进行博士后工作,利用低温电子顺磁共振(EPR)技术研究光合作用反应中心电子转移和催化机理。2004年以来在中科院化学所,主要从事自然光合作用和人工光合作用体系中电子转移和金属催化反应及其仿生模拟研究。主要业绩包括在人工光合作用方面首次合成出在结构和性能均与自然光合作用水裂解催化中心类似的仿生Mn4Ca-簇合物(见图1,C. Zhang, et al, Science 2015, 348, 690-693);在自然光合作用方面成功预测出光合水裂解催化中心关键辅基Ca的结合模式(C. Zhang, et al, Chinese Science Bulletin 1999, 44,2209-2215),并首次提出强氢键调控光系统II原初光化学反应和水催化裂解过程的调控机理(见图2,C. Zhang, Biochim. Biophys. Acta 2007, 1767, 493-499)。
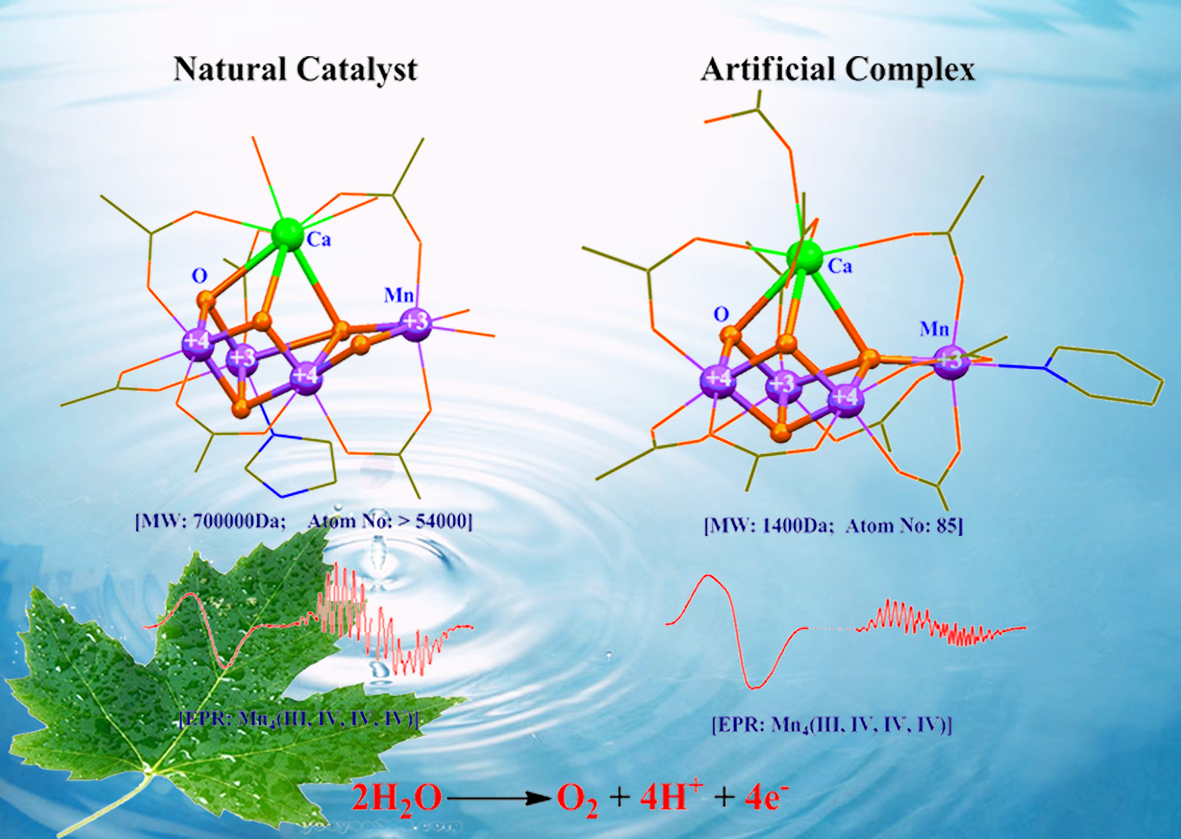
图1 自然光合作用和人工光合作用 的Mn4Ca-cluster的结构和性能比较
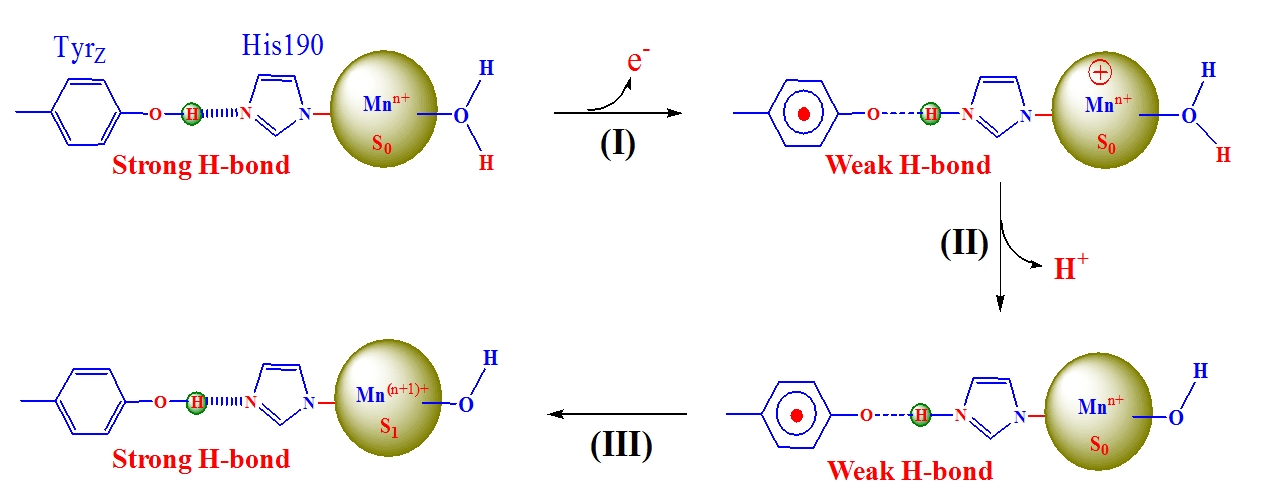
图 2光系统II中强氢键调控水裂解的机理模型
研究方向和兴趣:
一、人工光合作用:光合作用水裂解催化中心(又称放氧中心,简称OEC)是自然界唯一能利用太阳能高效、安全将水裂解,获得电子、质子的生物催化剂。借鉴光合作用的OEC,制备稳定、高效、廉价仿生水裂解催化剂,实现太阳能驱动人工水裂解, 获取电能或氢能是人工光合作用的研究目标。前期我们已在这方面取得突破,今后拟开展更深入的研究。
二、模拟固氮酶:固氮酶催化中心FeMo-co是自然界唯一能在常温、常压下将氮气还原为氨气的生物催化剂。人工模拟FeMo-co研究具有重要的科学意义和应用价值,但也是很具挑战性的科学难题,目前这方面研究尚未取得突破。
代表性论文:
- C. Zhang*, C. Chen, H. Dong*, J. Shen, H. Dau*, J. Zhao, A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis, Science, 2015, 348: 690-693.
- C. Chen, Y. Chen, R. Yao, Y. Li, C. Zhang*, Artificial Mn4Ca-cluster with exchangeable solvent molecules mimicking the oxygen-evolving center in photosynthesis, Angew. Chem. Int. Ed. 2019, 58: 3939-3942.
- C. Zhang*, T. Kuang*, A new milestone for photosynthesis, National Science Review 2018, 5: 444-445.
- C. Chen, Y. Li, G. Zhao, R. Yao, C. Zhang*, Natural and artificial Mn4Ca cluster for the water splitting reaction, ChemSusChem 2017, 10: 4403-4408.
- W. Chang, C. Chen, H. Dong*, C. Zhang*, Artificial Mn4-oxido complexes mimic the oxygen-evolving center in photosynthesis, Science Bulletin 2017, 62: 665-668.
- C. Zhang*, From natural photosynthesis to artificial photosynthesis, Sci. Sin. Chim. 2016, 46: 1101-1109. (in Chinese)
- C. Zhang*, The first artificial Mn4Ca-cluster mimicking the oxygen-evolving center in photosystem II, Science China, Life Sciences 2015, 58: 816-817.
- C. Chen, C. Zhang*, H. Dong*, J. Zhao, Artificial synthetic MnIVCa-oxido complexes mimic the oxygen-evolving complex in photosystem II, Dalton Trans. 2015, 44: 4431-4435.
- C. Chen, C. Zhang*, H. Dong*, J. Zhao, A synthetic model for the oxygen-evolving complex in Sr2+-containing photosystem II, Chem. Commun. 2014, 50: 9263-9265.
- L. Wang, C. Zhang*, J. Zhao, Location and function of the high-affinity chloride in the oxygen-evolving complex—implications from comparing studies on Cl-/Br-/I- substituted photosystem II prepared using two different methods, J. Photochem. Photobiol. B 2014, 138: 249-255.
- Y. Wang, C. Zhang*, L. Wang, J. Zhao, Assignment of the μ4-O5 atom in catalytic center for water oxidation in photosystem II, Chinese Science Bulletin 2013, 58: 3213-3216.
- Y. Ren, C. Zhang*, J. Zhao,Substitution of chloride by bromide modifies the low-temperature tyrosine Z oxidation in active photosystem II, Biochim. Biophys. Acta 2010, 1797: 1421-1427.
- H. Bao, C. Zhang*, Y. Ren, J. Zhao,Low-temperature electron transfer suggests two types of Qa in intact photosystem II, Biochim. Biophys. Acta 2010, 1797: 339-346.
- H. Bao, C. Zhang*, K. Kawakami, Y. Ren, J. Shen, J. Zhao, Acceptor side effects on the electron transfer at cryogenic temperatures in intact photosystem II, Biochim. Biophys. Acta 2008, 1777: 1109-1115.
- C. Zhang* Low-barrier hydrogen bond plays key role in active photosystem II ¾ a new model for photosynthetic water oxidation, Biochim. Biophys. Acta 2007, 1767: 493-499.
- C. Zhang*, Interaction between tyrosineZ and substrate water in active photosystem II, Biochim. Biophys. Acta 2006, 1757:781-786.
- C. Zhang, A. Boussac, A. W. Rutherford, Low Temperature Electron Transfer in Photosystem II: a Tyrosine Radical and Semiquinone Charge Pair. Biochemistry 2004, 43: 13787-13795.
- C. Zhang, S. Styring, Formation of split EPR signals in photosystem II suggests that tyrosine z can be photooxidized at 5K in the S0 and S1 states of the oxygen-evolving complex. Biochemistry 2003, 42: 8066-8076.
- C. Zhang, J. Pan, L. Li, T. Kuang, New structure model of oxygen-evolving center and mechanism for oxygen evolution in photosynthesis, Chinese Science Bulletin 1999, 44: 2209~2215.




